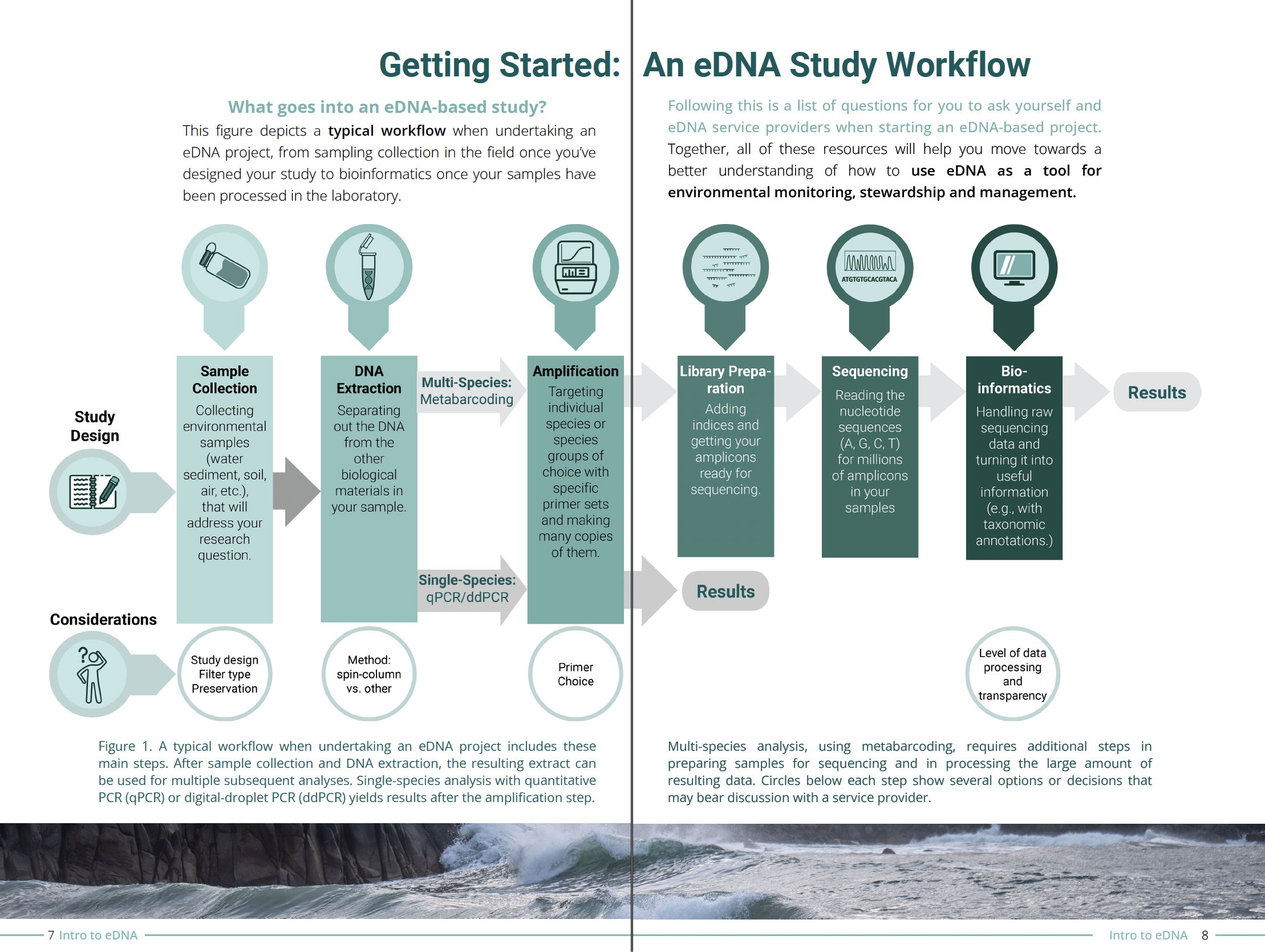
What's Happening Archive
My name is Ryan Moinazad, and this summer I had the incredible opportunity to join NOAA’s Ocean Molecular Ecology group as an undergraduate research volunteer. I’m a rising sophomore at the University of Southern California, double majoring in Biological Sciences and Legal Studies, and I also assist with research at the Natural History Museum of Los Angeles County (NHMLAC). My summer project, a continuation of my work at NHMLAC, focused on a long-standing challenge in science: how to get usable DNA out of specimens preserved in formalin.
Formalin, a diluted form of formaldehyde, has been widely used for specimen preservation because of how well it preserves their shape and form. Museum collections across the world have historically used formalin to preserve samples. The tradeoff is that while formalin preserves the physical structure of specimens exceptionally well, it often degrades their DNA. This damage makes it extremely difficult to recover genetic material, rendering many otherwise valuable collections challenging, or even impossible, to use for molecular research.
Recent research has shown promising ways to recover usable DNA from vertebrate specimens stored in formalin. My project extended this work to formalin-preserved plankton samples collected during the 2020 Washington Ocean Acidification Center (WOAC) cruises using net tows, a common method that involves dragging fine-meshed nets through the water to collect small, planktonic organisms.I compared two different methods for extracting DNA. The key difference in the methods was the chemical lysis solution used to break open the specimens' cells and release their DNA. For each method, samples were removed from their formalin fixation, washed, filtered, and then blended to create an evenly mixed sample. I found that a gentler buffer, closer to neutral pH, extracted ten times more DNA than a stronger, more basic buffer. After extraction, the DNA was treated with an enzyme-based “repair kit” that helps reverse some of the chemical changes caused by formalin.

The next step, which will continue this fall, is to sequence the extracted DNA. Traditional species counts have already been completed for each net tow sample, so comparing the genetic data to these visual identifications will provide a more comprehensive picture of plankton diversity and abundance. This comparison will also help validate the effectiveness of DNA extraction methods for formalin-preserved samples.
Overall, my time with the OME group has been incredibly impactful in both allowing me to grow my skills in research and better understand the invaluable work that happens at NOAA overall. The team here is truly incredible, and I’m grateful for the opportunity to contribute to work that could help unlock the genetic secrets of decades-old marine archives.
My name is Dwan Jackson, and I am a MSc student from Jackson State University interning in the PMEL Ocean Molecular Ecology (OME) group for the summer. My program, Center for Coastal and Marine Ecosystems-II (CCME-II), funds a NERTO (NOAA Experiential Research and Training Opportunities) internship for students to further their research abilities and gain experiences highly sought after within NOAA. In collaboration with OME, my MSc thesis focuses on the validation and application of a quantitative PCR (qPCR) assay to detect Dungeness crab in hopes of bolstering current crab monitoring efforts. Dungeness crabs are an important component of the coastal food web and the most valuable single-species fishery along the US West Coast. Understanding their future population size through larval counts helps to promote data-driven management efforts. Similar to a COVID-19 qPCR test, our assay targets Dungeness crab DNA sequences specifically, providing a sensitive, accurate, and quantitative method to detect Dungeness crab in the environment. The quantitative aspect of this assay allows for the exploration of our data to determine if there is a relationship between DNA concentration and the number of Dungeness crabs in the samples. The relationship allows for the potential to estimate larval numbers and expand population assessments in scope and scale without the time and labor of individual counting.
This project has granted me a variety of hands-on NOAA research opportunities this summer. Before collecting samples, we had to validate the assay performance, which involved both computer design of the assay and laboratory work using synthetic Dungeness crab DNA to test the assay sensitivity. This hands-on testing was invaluable, allowing me to master techniques like using a multi-channel pipette and a 384-well plate. Once we got to a good place in our assay validation testing, we coordinated with the PMEL Carbon group and the Pacific Northwest Crab Research Group (PNWCRG) to pair manual counts of crab larvae with the quantifiable DNA we can detect in the overlying water. These kinds of tests allow us to understand the relationship between the number of crab larvae and the amount of DNA they shed into the environment.
We began with previously collected Dungeness crab larvae collected by the PMEL Carbon Group from net tows along the US West Coast in 2016. Though these provided a few data points for comparison, we wished to broaden and strengthen our correlation between organism count and released DNA by adding additional range and replication. Thus, we partnered with the PNWCRG to incorporate those additional crab larvae samples.

Schematic of a light trap made of standard materials. Credit: PNWCRG
Our work with PNWCRG allowed us to sample water and larval crab specimens in Anacortes, WA. We joined Sarah Grossman, an environmental specialist with the Swinomish Indian Tribal Community, as she collected and counted the crabs from light traps. A light trap is an active sampler that depends on the larvae’s attraction toward the light source and traps them through funnel constriction points to prevent escape. During our collection, we were able to collect some of the Dungeness crabs and preserve them in buffered ethanol to add data for quantification purposes from controlled experiments with precise crab counts in jars. Additionally, while the light trap was pulled from the water, we collected the water that poured from the traps, providing a paired environmental sample to understand the overall impact on DNA quantification with the potential for crab signal from the surrounding environment. We also collected water from the dock and down shore for eDNA analysis. I enjoyed our sampling time a lot, from seeing light trap recovery first hand, observing the other species that also enjoy the light traps, and collecting water during the very low tide.
My time here has been valuable to me in developing my skills as a scientist and becoming better at communicating my science.

Dwan Jackson and Han Weinrich filtering water samples collected from the light traps in Anacortes, WA. Credit: Mugdha Chiplunkar
This spring, scientists aboard the annual NOAA EcoFOCI Mooring Cruise, collected nine sea ice samples to assess the sea-ice algae community in the Bering Sea. The PMEL Ocean Molecular Ecology (OME) group will extract and sequence these samples, alongside open water environmental DNA samples, to examine the differences between ice algae communities and open water communities. This work will enhance our understanding of the role ice algae play in the ecosystem after the winter sea ice melts.
As part of the ongoing collaboration between OME and Ecosystem and Fisheries Oceanography Coordinated Investigations (EcoFOCI), Han Weinrich joined the annual mooring cruise on the NOAA Ship Oscar Dyson. The cruise departed from Kodiak, Alaska, and traveled through the Aleutian Islands into the Bering Sea, continuing north toward the edge of the seasonal Arctic sea ice. Each spring, the winter's sea-ice sheet melts and broken floes remain into the spring season, creating a dynamic, nutrient-rich environment that contributes to this biologically diverse ecosystem. The melting sea ice conditions offer scientists a valuable opportunity to study how this melting sea ice interacts with the surrounding ocean. During the cruise, the team sought out the ice edge and collected samples from three ice floes. In addition, at more than 70 sites, water column samples were collected using a CTD Niskin array for nutrients and other oceanographic parameters. This includes the collection of 180+ open ocean water samples filtered for eDNA.
Ice algae are microscopic algae that develop both inside the small water pockets of frozen sea ice and grow along the bottom of the ice. These communities, often dominated by diatoms, serve as primary producers and play a critical role at the base of the food web, supporting everything from invertebrates to marine mammals. While melting ice releases algae into the surrounding water and can be captured through standard eDNA sampling efforts, sampling the ice directly provides a detailed sampling of the ice algae communities. The presence of sea ice in the region provided the team with the unique opportunity to complete this work. To collect these ice samples, the NOAA Ship Oscar Dyson carefully navigated alongside small ice chunks. Using long poles, the science team and deck crew broke off chunks of the ice, secured them in a net, and brought them on board. The team then worked diligently to sample each chunk before setting the ice aside to melt so eDNA filtering could occur. The team gained considerable knowledge while troubleshooting this methodology, including the fact that some larger samples take over 25+ hours to melt!
In total, nine melted ice samples from three ice floes were successfully collected. These melted ice samples will allow us to discover more about Bering Sea ice algae diversity and the impact these communities have on the marine food web.

Sea ice collected during the 2024 Fall EcoFOCI Mooring Cruise. The brown discoloration on the sample is visible ice algae.
The 2021 West Coast Ocean Acidification (WCOA) cruise aimed to better understand how changing ocean chemistry affects marine communities along the U.S. West Coast, from Mexico to Canada. As part of this effort, researchers used plankton tows to capture detailed snapshots of phytoplankton and zooplankton species and their diversity. The Ocean Molecular Ecology (OME) group, in partnership with the Carbon Group, is working to compare these traditional plankton collections with environmental DNA (eDNA) sampling. This comparison is helping validate the use of eDNA for future ocean monitoring efforts.
To start this process, UW undergraduates Mugdha Chiplunkar and Karina Lai spent several weeks inventorying the extensive collection of preserved plankton samples from the cruise. They cataloged each sample in a spreadsheet, recording collection details (like date and location) and taking photos of each container from multiple angles. This organized inventory made the samples more easily accessible, as it was faster to locate and reference individual samples without having to dig through the entire storage cabinet.

The next phase involved carefully removing a small volume of ethanol, the preservative in the jars, from each sample. This ethanol contains traces of DNA from the organisms in the sample, so filtering and analyzing it can reveal information about community composition. The process wasn’t always easy; some jars were packed with plankton, which made drawing out the ethanol a slow and tricky task.
To estimate the volume of plankton in each jar, Mugdha and Karina, alongside OME researcher Shannon Brown, used weight displacement. First, they estimated the total volume of the ethanol and plankton together using a graduated cylinder. Then, the contents were poured through a sieve to collect the liquid separately from the plankton. Subtracting the liquid volume from the total volume helped them estimate the volume of the plankton only. This process was time-consuming and required a lot of stirring, especially when larger plankton slowed the draining process. Transferring samples was also messy which required extensive sterilization and definitely left the lab smelling distinctly of preserved plankton for days.

Karina Lai & Mugdha Chiplunkar busy at work during the weight displacement step
After volume estimation, the team took the previous aliquoted ethanol and filtered over 150 ethanol samples onto disc filters over the course of three days. These filters were then processed for DNA extraction, PCR-amplified for target genetic markers, and sent out for sequencing. In parallel, OME also extracted DNA directly from the plankton subsamples taken from the jars themselves. This provided an additional comparison point in the study.
Though labor-intensive, this work provided Mugdha and Karina with valuable hands-on experience in marine sampling, molecular techniques, and ecological research. Along the way, they encountered a fascinating array of plankton, and even discovered a few tiny squid hiding among the samples. Their efforts are helping NOAA and the OME team advance the use of eDNA to monitor the ocean’s changing ecosystems.
Environmental DNA - or eDNA - is, simply put, DNA that can be found in the environment, such as soil, sediment, water, and air. Organisms are constantly shedding parts of themselves - such as dead skin, mucous, or waste - into their surroundings. The DNA in this shed organic matter, along with organismal DNA from microscopic organisms such as protists and bacteria, makes up eDNA. When a sample of water is collected, scientists can analyze the eDNA within it to determine what species are or were recently present in those waters, even if those species are not observed with the naked eye.
The use of eDNA is becoming increasingly popular across the world, including in the Northeast Pacific Ocean region. Approaches to collect and detect eDNA are now providing rapid, accurate, and cost-effective means of identifying marine organisms - from marine mammals to fish, invertebrates, and harmful algal blooms - and are being used to build valuable baselines for marine biodiversity datasets. Despite the growing evidence of eDNA’s effectiveness in answering a wide range of research questions, its application in environmental management is still evolving.
There are many possible applications of eDNA to marine monitoring and management, including Marine Protected Area (MPA) and Indigenous Protected and Conserved Area (IPCA) monitoring and management; threatened species detection; invasive species detection; and environmental restoration. As marine managers and stewards gain a better understanding of eDNA technology, and as scientists learn more about community needs, this powerful tool will continue to become more effective for environmental management
Whether you’re a marine steward or manager who is new to eDNA or someone already familiar with the technology but eager to learn more, this ‘eDNA primer’ is designed to deepen your understanding of this innovative tool. This resource was developed based on community responses from the Mobilizing eDNA for Management in the Northeast Pacific webinar and workshop, as well as the related Using Environmental DNA for Monitoring and Stewardship in the NE Pacific webinar.
Partners: eDNA Collaborative, Hakai Institute, The Ocean Decade Collaborative Centre for the Northeast Pacific, Fisheries and Oceans Canada (DFO), NOAA ‘Omics, and McGill University’s Sunday Lab
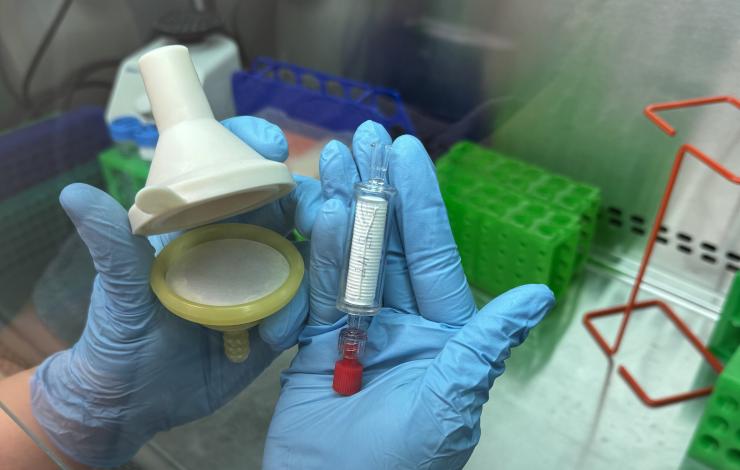
As part of the Ocean Molecular Ecology (OME) group’s effort to optimize eDNA collection techniques and broaden our sample collection capabilities at sea, we are conducting a study to compare a new filter produced by an American small business, the Smith-Root self preserving eDNA filter to our current filter, the Millipore sterivex capsule filter. The collection and filtration of eDNA samples at sea is limited in part by the technical skills required to filter and preserve these sensitive samples without contamination. With our current filter, the flammable preservative and low-temperature storage requirements also present a barrier to shipping and sample storage in some field applications.
The self-preserving filter, developed by a small business in Vancouver WA, is designed to capture and preserve eDNA from multicellular organisms. It has been found to perform as well as ethanol-based preservation for a 6 month period. Rather than a preservative, the Smith-Root filter utilizes a proprietary desiccating filter housing to preserve captured DNA and cells, eliminating the need for chemical preservatives. This design simplifies operation, requires less training, and streamlines shipping. However, these filters have not been tested against the sterivex filter, nor have they been tested on their ability to capture DNA from single-celled organisms. Our current study aims to address these gaps by testing the sterivex head-to-head against the Smith-Root filter using the same filter material and pore size.

For this study, Han Weinrich and Zack Gold collected water samples from local fresh and seawater sources alongside water from the Olympic Coast National Marine Sanctuary, then they filtered them in the lab - half with sterivex filters and half with the Smith-Root filters. To avoid any degradation of DNA before preservation, Han and Zack filtered the samples immediately after collection, resulting in several 12 hour days. DNA extractions at six time points, from one day to six months post-collection, will assess the preservation performance of the Smith-Root filter relative to the sterivex filter. Preliminary results from extractions conducted up to three months post-collection indicate that the Smith-Root filter preserves DNA comparably to the sterivex filter. Further analyzes will include sequencing the extractions to determine if both filters capture and preserve the same organisms at the same proportions - a critical metric for the OME as we aim to capture biodiversity at all levels of the ecosystem, from bacteria to whales.
If the Smith-Root filters perform comparably to the sterivex in meeting OME's eDNA objectives, they may offer an alternative to sterivex filters in certain field applications - saving time and space on research vessels while enabling eDNA monitoring in a broader range of environments. In addition, the National Aquatic eDNA Strategy seeks to advance eDNA sampling technologies by developing low-cost, high fidelity devices to enable large-scale collection. The Smith-Root filter could be employed in the development of a new eDNA sampler.

Since 2020, the Ocean Molecular Ecology (OME) group has collaborated with Ecosystem and Fisheries Oceanography Coordinated Investigations (EcoFOCI) to maintain a seawater environmental DNA (eDNA) time series in the U.S. Arctic. This fall, Shannon Brown participated in the annual EcoFOCI Fall Mooring cruise, which focuses on deploying and recovering oceanographic moorings and collecting shipboard biophysical samples at and between mooring locations. U.S. Arctic marine ecosystems are rapidly changing in response to climate change with accelerating ocean warming and sea ice loss leading to cascading ecosystem effects. OME leverages eDNA approaches to examine the regional biodiversity of microbes, phytoplankton, zooplankton, fishes, and mammals. By pairing eDNA analyses with physical and chemical measurements, these efforts establish critical baselines that support fisheries management and marine conservation. Additionally, in collaboration with the FWC Center for Red Tide Research, our eDNA samples contribute to studying harmful algal blooms (HABs), which can affect marine life and human industries in the region.
During this cruise, Shannon successfully filtered 110 eDNA samples collected from CTD Niskin bottles. The team also deployed an autonomous eDNA sampler at the M2 mooring site. Designed to collect samples biweekly for a year, this sampler had previously been deployed in the Chukchi Sea, where it successfully gathered 24 samples from September 2023 to July 2024. It was recovered in August by the RV Sikuliaq and immediately shipped from Nome, AK to Dutch Harbor, AK, to meet the Fall Mooring cruise.
Monitoring in the Arctic is challenging given its remoteness and the difficulty of navigating harsh sea ice and ocean conditions, making it impossible to manually sample for half the year. Autonomous eDNA samplers are an innovative solution, enabling year-round biodiversity sampling. Due to a limited number of samples and only a few deployment opportunities, we decided to collect one unit, turn it around in the field, and redeploy it for another year.

Redeploying the autonomous sampler highlighted the logistical complexity of Arctic fieldwork. Alaska, the largest U.S. state, lacks road connections between key locations like Nome and Dutch Harbor, requiring equipment to be transported by plane or boat. With only a week between cruises, the team opted to ship the sampler by air—a process fraught with delays. Unfortunately, after countless hours spent coordinating the unit transfer, the sampler missed the departure of the NOAA Ship Oscar Dyson departure date. Fortunately, Han Weinrich was still on the island and ready to receive the delayed unit. A few days after departing, the NOAA Ship Oscar Dyson returned briefly to Dutch Harbor, and the amazing crew retrieved the sampler with a small boat.
Once aboard, Shannon and the EcoFOCI team worked tirelessly to prepare the sampler for deployment within 48 hours. When a malfunction caused the unit to power down spontaneously, Shannon spent hours disassembling and reassembling components to diagnose the issue. With the help of Oscar Dyson’s Chief Engineer (a true MacGyver), foam ear plugs, and some new springs, a solution was found just three hours before the unit was successfully deployed!
Sadly, the sampler was prematurely recovered by a fishing vessel on November 24, just 11 weeks after redeployment. While it’s unclear if any samples were collected, the incident underscores the unpredictable nature of field research in the Arctic. Despite these challenges, this mission highlights the resilience and ingenuity of the scientific team. The data collected through these efforts will inform the sustainable management of Arctic marine ecosystems and improve our understanding of biodiversity in this rapidly changing region. Thanks to the crew of the NOAA Ship Oscar Dyson and the entire science party for a successful cruise!

Shannon Brown preparing an autonomous eDNA sampler for deployment on the NOAA Oscar Dyson. Credit: Mabel Baldwin-Schaeffer/NOAA Fisheries
The Phycological Society of America’s (PSA) joint meeting with the International Society of Protistology (ISOP) and International Society for Evolutionary Protistology (ISEP) brought together algae, phytoplankton and protist researchers from across the globe. During the meeting, research from carbon dioxide draw-down with kelp farms to important evolutionary questions of protists in the tree of life were presented to an international audience of academic, federal, state, and local government scientists.
Sam Setta presented on phytoplankton changes with temperature and acidification from the West Coast Ocean Acidification (WCOA) 2021 Cruise. During the meeting, Sam had the opportunity to connect with many scientists, including researchers from the Washington Department of Natural Resources and Shannon Point Marine Center at Western Washington University.
Following the conference, Sam flew to Savannah, Georgia for Dr. Holly Bik’s workshop “Telling Stories Through Data”. Unfortunately for workshop participants, this happened to coincide with Hurricane Debby’s arrival on the U.S. East Coast. While the workshop was originally planned to take place on Sapelo Island, evacuation orders meant the location changed to downtown Savannah, GA.
During the workshop (and Hurricane Debby), Sam learned new data visualization methods and best practices. In the afternoon sessions, Dr. Virginia Schutte led the group in a science communication component. Sam and workshop participants explored a variety of science communication platforms and best practices and brainstormed a future science communication plan. Graduate students to early career researchers participated in the workshop and learned data visualization and storytelling from each other while forming a community to rely on for tips and advice on “Telling Stories Through Data” in the future.
Through these two great experiences this summer, Sam was able to communicate with scientists on findings from the West Coast Ocean Acidification cruise, meet scientists with similar research, learn data visualization and science communication, and meet a wonderful group of early career researchers.

My name is Ella Crotty, and I am an undergraduate research intern through the NOAA Ernest F. Hollings Scholarship Program. I chose to work with OME for my internship because I didn't know very much about environmental DNA (eDNA), and I wanted to learn! eDNA refers to genetic material that organisms leave behind in the environment by shedding skin, releasing mucus, etc. It can be used to detect if species are present in an area of the ocean using a water sample. For my project, I worked with an automated sampler that sits in the water and collects eDNA samples at regular intervals by filtering water samples through super-fine filters.
My internship included fieldwork in the Olympic Coast National Marine Sanctuary (OCNMS). I went out to the northwest coast of Washington twice to help with sampler recovery and redeployment. I helped collect and filter eDNA samples on the boat, then after the sampler was recovered, I helped store the samples from the sampler, which had already been filtered underwater. We returned to land and spent a day sterilizing the sampler before redeploying it, which was a complicated process. I had never worked with such a big and complicated instrument before, and it was interesting to learn about the logistics of setting up and maintaining something like that without access to a full lab! We brought a lot of tools and extra parts, in case anything broke and needed to be fixed on the coast between boat days.
The main goal of my research project was to determine how the presence of different species in OCNMS changes when the amount of oxygen in the water is low. Low oxygen, also known as hypoxia, can cause stress and death in marine animals. Two mg/L of oxygen is often used as the upper limit of what counts as hypoxic. I compared the data from our eDNA samples, which told me which species were detected in the sanctuary on certain dates, with dissolved oxygen data sampled from CTD casts and moorings. In OCNMS during the summer, a process called coastal upwelling causes oxygen-poor deep water to move up from the bottom of the Pacific Ocean, which decreases oxygen levels near the coast. However, some species are more sensitive than others, and some, like larger fish, are mobile and can move away from the low-oxygen areas. So, our main research question was: which species stick around when the oxygen drops, and which can no longer be detected? This question will help us understand how seasonal changes in oxygen are impacting OCNMS waters.
It required quite a few stages of data analysis and quality-checking in order to make the environmental data and eDNA data compatible with each other. Using the programming language R, I cleaned the data and matched the species detections to the temperature and oxygen at the date and time when the eDNA samples were taken. I implemented a lot of checkpoints to ensure that the code was doing what I wanted it to. For example, I checked to make sure that everything was in the same time zone, but between the CTDs, mooring, eDNA sampler, and handwritten sample data, I had to be very vigilant about converting the time zones before combining data.
First, I focused on species that OCNMS and the coastal treaty tribes consider high priority for monitoring and management and that were detected at least 10 times. Of the 64 priority species, we detected 24 and four were detected at least 10 times. The two species with the most detection data were Pacific herring and the copepod Acartia longiremis. I didn't find any correlation between oxygen levels and the presence of these species, which suggests that seasonal hypoxia is not impacting these commonly detected taxa. Herring are known to be pretty hypoxia-tolerant, which agrees with my findings. I also investigated species outside the priority list by calculating a bunch of statistics and filtering for significant results. I found a few species that did have observable relationships with hypoxia, including two species of copepod, tiny crustaceans that are used as indicators of ecosystem health. Through this internship, I developed a code workflow that combines environmental data with eDNA data, which is useful as we continue to study how different species are affected by environmental conditions.

As the Ocean Molecular Ecology (OME) group works to make our science and lab practices open and accessible, we have prioritized standardizing and sharing our environmental DNA sample collection and processing protocols to a public repository. The Better Biomolecular Ocean Practices (BeBOP), a U.N. Decade Endorsed project under Ocean Biomolecular Observing Network (OBON), pioneered a Minimum Information about an Omics Protocol (MIOP) format to better compare practices and integrate data generated when studying ocean life. BeBOP formatted protocols are accessible, detailed, traceable, machine-readable, and standardized. Importantly, they are designed to provide the reader with all the information needed to replicate a protocol from supplies purchasing and troubleshooting to the method background and a step-by-step procedure.
In the last four years, OME has collected over 2,800 eDNA samples from coastal stations off the western coast of North America in the Northeastern Pacific Ocean, Bering Sea, and Arctic Ocean. Since 2023, we’ve extracted over 1,200 samples and sequenced/processed over a thousand. As we pushed through our backlog, we fine-tuned our standard operating procedures for all in-house lab work, including eDNA collection, extraction, and PCR amplification. Our published protocols are written for reproducibility so a new lab could replicate our efforts. We aim to continue our efforts to encompass all field and lab protocols performed by OME over the next year. These protocols are archived, version-controlled, and citable in Zenodo repositories.