 , H
, H S,
Fe
S,
Fe , etc.) can mix and react with electron
acceptors in circulating seawater (O
, etc.) can mix and react with electron
acceptors in circulating seawater (O ,
SO
,
SO
 ,
NO
,
NO
 ),
and it is within this zone that fluid-microbe interactions will be most important
(see discussions in Jannasch
(1995) and Karl
(1995)). If heat, volatiles and metals are rapidly exchanged in the very shallow
subseafloor, then size of habitat, opportunity for microbiological growth, and
impact of microbes on fluid chemistry are all diminished. The chemical composition
of the fluids during the post-eruption period is an important factor influencing
microbial growth and colonization (Holden
1996), and is ultimately linked to the details of the volcanic event.
),
and it is within this zone that fluid-microbe interactions will be most important
(see discussions in Jannasch
(1995) and Karl
(1995)). If heat, volatiles and metals are rapidly exchanged in the very shallow
subseafloor, then size of habitat, opportunity for microbiological growth, and
impact of microbes on fluid chemistry are all diminished. The chemical composition
of the fluids during the post-eruption period is an important factor influencing
microbial growth and colonization (Holden
1996), and is ultimately linked to the details of the volcanic event.
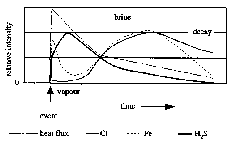
Figure 5: Response of hydrothermal systems to a volcanic event. Relative intensity of heat flux (dot-dashed line) and vent fluid concentrations of chloride (thin line), iron (dotted line), and hydrogen sulphide (thick line) over time. Systems evolve from a vapour-dominated, high heat flux stage accompanied by phase separation, through a transition to brine-dominated discharge, and eventually decay back toward zero heat flux and seawater composition. Our observations suggest high Fe concentrations in immediate post-eruptive fluids. Response model is based on this work, Butterfield & Massoth (1994), Von Damm et al. (1995), Lupton (1995), and Baker (1995).
The two very different types of diffuse fluids seen at CoAxial (oxidized, Fe-rich
fluids at Flow, and more reducing H S-rich
fluids at Floc) can be explained by a general model of how vent fluids evolve
following a volcanic event (figure 5), taking
into account the likely differences in the location of the heat source within
the oceanic crust (figure 6). Immediately following
a volcanic eruption or dyke injection, heat flux increases greatly, triggering
phase separation and preferential venting of the more buoyant vapour phase.
Continuous venting of brines observed in some systems means the conjugate brine
phase must be temporarily retained around the heat source (by virtue of higher
density or other physical properties), while higher-enthalpy vapour-rich fluids
with low chlorinities remove heat, volatiles (H
S-rich
fluids at Floc) can be explained by a general model of how vent fluids evolve
following a volcanic event (figure 5), taking
into account the likely differences in the location of the heat source within
the oceanic crust (figure 6). Immediately following
a volcanic eruption or dyke injection, heat flux increases greatly, triggering
phase separation and preferential venting of the more buoyant vapour phase.
Continuous venting of brines observed in some systems means the conjugate brine
phase must be temporarily retained around the heat source (by virtue of higher
density or other physical properties), while higher-enthalpy vapour-rich fluids
with low chlorinities remove heat, volatiles (H S,
CO
S,
CO , He and H
, He and H from magmatic degassing and water-rock interaction partition into the vapour
phase) and metals (figure 6). Iron concentration
during the vapour-dominated period will be a function of temperature, pH, Cl
concentration and possibly reaction kinetics, and cannot be assumed to be simply
proportional to Cl (Seyfried
& Ding 1995). As heat is removed and the system cools, phase separation
slows and stops, and the fluids go through a transition from vapour-dominated
to brine-dominated (chloride and metals increase while volatiles decrease).
Brine content in the vented fluids may reach a peak and then decline as heat
from the system runs out and fluid compositions decay back toward seawater.
from magmatic degassing and water-rock interaction partition into the vapour
phase) and metals (figure 6). Iron concentration
during the vapour-dominated period will be a function of temperature, pH, Cl
concentration and possibly reaction kinetics, and cannot be assumed to be simply
proportional to Cl (Seyfried
& Ding 1995). As heat is removed and the system cools, phase separation
slows and stops, and the fluids go through a transition from vapour-dominated
to brine-dominated (chloride and metals increase while volatiles decrease).
Brine content in the vented fluids may reach a peak and then decline as heat
from the system runs out and fluid compositions decay back toward seawater.
Figure 6: Chemical and microbial processes in a diffuse upflow zone. Injection of a dyke results in delivery of reduced volatiles and metals through outgassing and water-rock interaction. Phase separation partitions volatiles and some metals into vapour phase, and brines accumulate around heat source. Thermal and redox gradient provides a zone for chemical and microbial oxidation of reduced gases and metals as circulating seawater is entrained (microbial methanogenesis and sulphur reduction are known to occur at 110°C).
The time scale in figure 5 is relative to the
size of the volcanic event. Vents at 9°46.5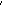 N
on the EPR showed an increase in chlorinity but were still below seawater chlorinity
three years after a seafloor eruption (Von
Damm et al. 1995). North Cleft segment diffuse and high-temperature
fluids reached the brine emission stage at least three years after the volcanic
event (Butterfield
& Massoth 1994). High-temperature fluids are still evolving at North Cleft
(Butterfield, unpublished data) and it is not clear how long the decay phase
may last. We propose that cooling and evolution of fluid chemistry was so fast
at the Flow site that we missed the vapour-dominated venting period entirely.
It took only 3-12 weeks to reach the brine stage at Flow, and venting was virtually
exhausted after one year, while venting of low-chlorinity fluids continued for
at least a year at Floc.
N
on the EPR showed an increase in chlorinity but were still below seawater chlorinity
three years after a seafloor eruption (Von
Damm et al. 1995). North Cleft segment diffuse and high-temperature
fluids reached the brine emission stage at least three years after the volcanic
event (Butterfield
& Massoth 1994). High-temperature fluids are still evolving at North Cleft
(Butterfield, unpublished data) and it is not clear how long the decay phase
may last. We propose that cooling and evolution of fluid chemistry was so fast
at the Flow site that we missed the vapour-dominated venting period entirely.
It took only 3-12 weeks to reach the brine stage at Flow, and venting was virtually
exhausted after one year, while venting of low-chlorinity fluids continued for
at least a year at Floc.
We attribute this post-eruptive fluid evolution to cooling of magma injected into the permeable upper layer of oceanic crust. In the absence of recent volcanic perturbations, it appears that low-chlorinity volatile-rich fluids are associated with high heat flux systems driving deeper phase separation (e.g. Endeavour Main Field), while brine-dominated fluids are associated with lower heat flux systems (e.g. Cleft segment) (see heat flux estimates in Baker 1995). The model depicted in figure 5 may apply in a general sense to all hydrothermal systems insofar as intensity of heat output over time should correlate with fluid chemistry. The Source vent area at CoAxial could be interpreted to be in the decay phase in figure 5. So far, no hydrothermal system has been sampled before and after a known volcanic event, but we propose that the model for post-eruptive fluid evolution would apply in an established hydrothermal system as well as in areas with no pre-existing, active hydrothermal system.
Figure 7: Photograph of halite coating on basalt within a cavity from
Alvin rock sample 2672-7, recovered in October from the crest of the July 1993
lava flow. Halite coating is intergrown with anatase (TiO ),
boehmite (AlO(OH)), and rare sphalerite (ZnS) needles (prominent in this photograph).
Interpretation is eruption caused by immediate phase separation and halite precipitation,
followed by a high-temperature reaction period. Mineral compositions were determined
by XRD and EDS-SEM at the Geological Survey of Canada in Ottawa.
),
boehmite (AlO(OH)), and rare sphalerite (ZnS) needles (prominent in this photograph).
Interpretation is eruption caused by immediate phase separation and halite precipitation,
followed by a high-temperature reaction period. Mineral compositions were determined
by XRD and EDS-SEM at the Geological Survey of Canada in Ottawa.
The Flow site is located at the distal end of the dyke injection (Dziak et al. 1995) and was characterized by shallow crustal activity relative to the Floc site ( Schreiner et al. 1995 ) and a pillow-lobate eruption (Embley et al. 1995). The chemistry of the event plumes, vent fluids and basalt alteration products at Flow site is consistent with an initial high-temperature venting period lasting less than three weeks after the end of the eruption, followed by rapid cooling of the lava mound and underlying dyke. The large inventory of Fe and Mn and the presence of ZnS particles in the event plumes (Massoth et al. 1995) and the high particulate Cu/Fe ratios in event plume A (0.002-0.005, compared to less than 0.0016 in the diffuse fluid-dominated chronic plumes) indicate that a high-temperature fluid contributed to event plume formation. (Plume particle chemistry determined by XRF.) Many of the freshly erupted basalt samples recovered showed evidence of an initial high-temperature reaction period: halite coatings precipitated by direct contact of seawater with very hot rock; fresh needles of sphalerite and small crystals of pyrite and chalcopyrite lodged on top of halite and other surfaces (figure 7), indicating flow of fluids from a hot (greater than 350°C) reaction zone; and pervasive staining consisting of oxides (amorphous iron oxide, boehmite, anatase), kaolinite and chlorite, indicating temperatures of 250°C and up to greenschist conditions. The precipitation of halite coatings on altered glass surfaces is consistent with heating seawater to temperatures of 440°C at 235 bar local seafloor pressure ( Bischoff & Pitzer 1989 ) and represents a short-term storage of chloride. Vapour-dominated fluids must have vented when halite was precipitating. Continued circulation of warm fluids through the lava flow should dissolve the halite and increase the chlorinity of vented fluids. Some of the halite coatings on basalts collected in October 1993 showed surface textures indicative of partial dissolution.
Figure 8: Geologic interpretation of CoAxial hydrothermal evolution, with depth below sea level in meters on vertical axis and latitude (°N) on horizontal axis. At the distal end of the dyke injection, heat is removed rapidly by formation of event plumes and circulation of seawater through the permeable lava mound, while a larger and deeper heat source near the magma supply continues to discharge for several years and provides a habitat for microbial communities. See discussion.
At the Floc site, we propose that the heat source was deeper, larger and not
directly exposed to ambient seawater (figure 8),
allowing the vapour-dominated period to last for at least one year. At two years
after the event, fluid chlorinities were nearly identical to seawater, suggesting
that this site was making the transition toward brine-like composition. We predict
that Floc site fluids will evolve to become brine-dominated, eventually becoming
totally depleted in H S and dominated
by iron, as seen at North Cleft and at Flow.
S and dominated
by iron, as seen at North Cleft and at Flow.
Subseafloor chemical and microbial oxidation of sulphide has the potential
to generate significant quantities of particulate elemental sulphur, which we
have observed in direct association with biogenic particles in the plume over
Floc. Our data and general model strongly suggest that methane (possibly from
subseafloor methanogens) and biogenic particles vented from post-eruptive vapour-dominated
diffuse vents contribute significantly to the high CH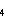 /Mn
and particulate S/Fe ratios observed in hydrothermal plumes over magmatically
active ridge segments (Lupton
et al. 1993).
/Mn
and particulate S/Fe ratios observed in hydrothermal plumes over magmatically
active ridge segments (Lupton
et al. 1993).
We hypothesize that nearly all of the potential heat to be extracted at the
Flow site is contained in the seafloor lava flow and shallow underlying dyke
and conduits, and that a significant part of this heat was extracted and formed
event plumes during the eruptive event. The decline in observable fluid flux
and maximum measured vent fluid temperature from 51°C on 1 August 1993,
to 36°C in mid-October, to only 9°C in July 1994 shows that the heat
source at the Flow site was nearly exhausted within a year. Using the volume
of extruded lava (5.4 × 10 m
m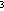 )
estimated by Chadwick et al. (1995) and the basalt properties found in
Sleep
et al. (1983), we calculate that the heat available in the lava mound
through latent heat of crystallization and cooling to ambient seawater temperatures
is 2.7 × 10
)
estimated by Chadwick et al. (1995) and the basalt properties found in
Sleep
et al. (1983), we calculate that the heat available in the lava mound
through latent heat of crystallization and cooling to ambient seawater temperatures
is 2.7 × 10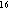 J. The estimate of Baker
et al. (1995) for the heat contained in the three event plumes (1.8
× 10
J. The estimate of Baker
et al. (1995) for the heat contained in the three event plumes (1.8
× 10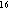 J) is equivalent to 2/3 of the
heat within the lava mound. (Diffuse venting was seen north and south of the
lava mound, and consideration of the potential volume of dyke beneath the entire
area of known venting at Flow site (1 m × 8 km × 2 km) increases the
available heat by a factor of three.) There is no evidence for hydrothermal
discharge at the Flow site immediately preceding the 1993 eruption, so it is
difficult to invoke a significant shallow crustal hydrothermal fluid reservoir,
as required in some event plume models (Cann
& Strens 1989). High-temperature sulphide minerals clearly precipitated
from the early fluids exiting the lava mound (figure
7) and therefore provide a source for the sulphide minerals observed in
the event plumes. Because the CoAxial volcanic event was pulsed and episodic
over several weeks, the initial penetration of a dyke in the eruption area creates
a high-temperature reaction zone which could be disrupted and emptied during
later eruptive pulses. The very large permeability required to generate event
plumes by dyke injection (Lowell
& Germanovich 1995) is provided here by the presence of lava at, and directly
below, the seafloor. The published plume data (Baker
et al. 1995; Cannon
et al. 1995; Lavelle
1995; Lupton
et al. 1995; Massoth
et al. 1995) and our evidence for very short-lived, high-temperature
venting support the hypothesis that the observed CoAxial event plumes resulted
directly from rapid high-temperature water-rock interaction during the seafloor
eruption and shallow dyke injection. The presence of erupted lava mounds near
the North Cleft megaplume site provides some support that this is a general
mechanism of event plume formation but questions remain regarding the relationship
of event plume chemistry to event plume formation mechanisms.
J) is equivalent to 2/3 of the
heat within the lava mound. (Diffuse venting was seen north and south of the
lava mound, and consideration of the potential volume of dyke beneath the entire
area of known venting at Flow site (1 m × 8 km × 2 km) increases the
available heat by a factor of three.) There is no evidence for hydrothermal
discharge at the Flow site immediately preceding the 1993 eruption, so it is
difficult to invoke a significant shallow crustal hydrothermal fluid reservoir,
as required in some event plume models (Cann
& Strens 1989). High-temperature sulphide minerals clearly precipitated
from the early fluids exiting the lava mound (figure
7) and therefore provide a source for the sulphide minerals observed in
the event plumes. Because the CoAxial volcanic event was pulsed and episodic
over several weeks, the initial penetration of a dyke in the eruption area creates
a high-temperature reaction zone which could be disrupted and emptied during
later eruptive pulses. The very large permeability required to generate event
plumes by dyke injection (Lowell
& Germanovich 1995) is provided here by the presence of lava at, and directly
below, the seafloor. The published plume data (Baker
et al. 1995; Cannon
et al. 1995; Lavelle
1995; Lupton
et al. 1995; Massoth
et al. 1995) and our evidence for very short-lived, high-temperature
venting support the hypothesis that the observed CoAxial event plumes resulted
directly from rapid high-temperature water-rock interaction during the seafloor
eruption and shallow dyke injection. The presence of erupted lava mounds near
the North Cleft megaplume site provides some support that this is a general
mechanism of event plume formation but questions remain regarding the relationship
of event plume chemistry to event plume formation mechanisms.
Acknowledgements. We thank the ROPOS and Alvin Deep Submergence teams, Ed Baker and John Lupton for comments on the manuscript, Joe Cann for editing, Ryan Whitney and Martha Jackson for word processing, Aries Galindo for graphics, and My Nguyen for assistance with chemical analysis. This research was supported by the NOAA VENTS Program, the National Undersea Research Program, and the U.S. National Science Foundation. PMEL contribution number 1731. JISAO contribution number 362.
Return to previous section or go to References and Discussion