Efforts to model the eastern Bering Sea ecosystem were reviewed by Francis et al. (1999). Models of ecosystem dynamics of the southeastern Bering Sea shelf span the full spectrum from conceptual models (e.g., the OCH, Hunt et al., 2002; the Trophic Cascade Hypothesis, Merrick, 1995; NRC 1996), to trophic or food web models (e.g., Laevastu and Larkins, 1981; Trites et al., 1999), to models that explicitly include links between specific ecological processes, such as physical processes and primary/secondary production (Hood, 1999; Walsh and McRoy, 1986). After providing some essential background information on the ecosystem, we focus on the OCH conceptual model. This model both encompasses most of the previous results and ideas regarding how the ecosystem of the southeastern Bering Sea functions, and it extends the conceptual linkages of processes, which were initially developed in the PROBES study (Walsh and McRoy, 1986).
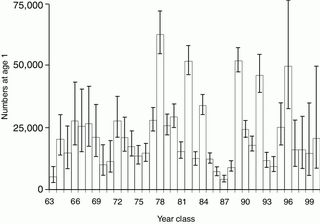
Currently, pollock is the most abundant species harvested in the Bering Sea, accounting for >65% of the total groundfish biomass; during the 1980s their total biomass exceeded 20 million metric tons (Napp et al., 2000). The biomass trends in the eastern Bering Sea (1979 to 1998: Schumacher et al., 2003) show that while the total biomass of pollock in the 1990s was less than in the 1980s, they still dominate biomass in any of the trophic guilds which include marine birds, mammals, other fishes and crabs. Walleye pollock is a nodal species in the food web (NRC Report, 1996) with juveniles being the dominant prey of fishes (including adult pollock), seabirds, and marine mammals (Springer and Byrd, 1989; Livingston, 1993). The abundance of pollock is determined by episodic occurrence of strong year-classes. Such a strong year-class occurred in 1978, the remaining year-classes fluctuating about the long-term mean abundance (Fig. 30.9).
Figure 30.9: Time series of year class abundance of age-1 pollock. Error bars represent ~95% confidence interval. (From Ianelli et al., 2001.)
The observational basis for the OCH includes many of the elements of the pathway model, including sea ice, wind generated turbulence, water column temperature and chlorophyll fluorescence (Fig. 30.10). The observations of biota included zooplankton, jellyfish, and four species of fish, marine mammals and sea birds. After an analysis of these data, Hunt et al. (2002) formulated the OCH. The OCH recognizes that late retreat of sea ice with the attendant cold temperatures in the water column and early, short phytoplankton blooms are hallmarks of cold regimes. The associated impacts on biota include reduced survival of fish eggs (Blood, 2002) and diminished production of zooplankton prey for larval fish. Under this set of conditions, the hypothesis predicts that recruitment of pollock will be nominal or weak, and strong year-classes are not expected. Bottom-up processes dictate the flow of energy through the ecosystem during a cold regime (Fig. 30.11). Cold water-column temperatures can directly impact distributions of some forage fish species. The OCH allows that pinnipeds and piscivorous seabirds may thrive, even under cold conditions, if the population centers of forage fish change and become more available as prey. During years when sea ice is either not present or retreats before there is sufficient net short wave radiation to initiate a bloom, the spring bloom occurs later than during the cold regime, and water column temperatures are warmer. Under this set of conditions, the spring bloom is expected to be prolonged and zooplankton production is expected to be strong, resulting in readily available prey for larval and juvenile fish. The potential then would be high for strong year-classes of pollock and other piscivorous fish (Fig. 30.11).
Figure 30.10: A synthesis of physical phenomena that help dictate the timing of the spring phytoplankton bloom of the southeastern Bering Sea shelf. The top panel includes the timing of the last retreat of sea ice, the timing of the shift between winter and summers wind conditions, and their effect on the timing of the spring bloom over the southern portion of the middle domain. Note that ice-related or early blooms occur in years when the ice retreat comes in mid March or later. In 1975 there was an ice-related bloom in May. The date of the last winter storm was defined as when the wind speed cubed fell below 2500 m![]() s
s![]() for the summer. The winds were measured at St. Paul Island. (From Hunt et al., 2002.) The lower panel shows the relationship between ice retreat and date of last winter storm with the years divided into regimes. If the relationship between timing of ice retreat and spring phytoplankton bloom hold, then the timing of the spring bloom can be inferred for each regime.
for the summer. The winds were measured at St. Paul Island. (From Hunt et al., 2002.) The lower panel shows the relationship between ice retreat and date of last winter storm with the years divided into regimes. If the relationship between timing of ice retreat and spring phytoplankton bloom hold, then the timing of the spring bloom can be inferred for each regime.
Figure 30.11: Schematic of the OCH. The number of pollock recruits affects not only pollock population dynamics, but also the availability of age-1 pollock to predators such as marine birds and mammals. Top Panel: In a cold regime, copepod production is limited by water temperature and larval/juvenile pollock survival is prey limited. Upper Middle Panel: At the beginning of a warm regime, copepods flourish and provide ample food resources to support strong survival of larval and juvenile pollock; juvenile pollock survival is high because there are few large piscivorous fish to consume them. Lower Middle Panel: In a warm regime, the production of copepod food for larval and juvenile pollock remains strong, but survival of juveniles and recruitment is limited to cannibalism from the growing biomass of adult pollock and other predators such as Pacific cod and arrowtooth flounder. Bottom Panel: At the beginning of a cold regime, copepod production is limited and larval and juvenile pollock are food limited. In addition, cannibalism by adult pollock and other predators further limits recruitment. Thus, there are asymmetries in how pollock recruitment will respond to changes from a cold to a warm regime versus from a warm to a cold regime.
There are years when the observed regime conditions (e.g., 1976 – cold with low recruitment, 1996 – warm with high recruitment) and estimated pollock production fit the outcomes predicted by the OCH; however, there are also years when the fit is poor (e.g., 1987 – warm with low recruitment, 1992 – cold with high recruitment). The history of the predator field (i.e., top-down process) on young pollock, therefore, must also be taken into account. The marked impact of adult pollock on age-1 and younger fish, together with other fish (e.g., arrowtooth flounder and Pacific cod) is well documented (Livingston et al., 1999; Livingston and Methot, 1998). The OCH weaves in such biological mechanisms as follows. When there is a sequence of warm regime years, recruitment is above average and the populations of adult predatory fish will eventually increase to a point where the control of future year-class strength is mainly a top-down process. This switch to top-down dominance may have occurred for the pollock population in the Gulf of Alaska (Bailey, 2000). There, as the arrowtooth flounder population markedly increased, the pollock population underwent a severe decline and the "critical period" for determining year-class strength switched from the egg and larval stages to the juvenile period. The hypothesis predicts that as predation becomes greater, the abundance of young pollock and forage fish decline and zooplankton become available for other populations (e.g., jellyfish, salmon, baleen whales). In addition, the reduction in abundance of forage fish could cause declines in populations and/or productivity of pinnipeds and piscivorous seabirds (Hunt and Stabeno, 2002).
While decadal time scale or regime shifts have been identified for the Bering Sea (e.g., Stabeno et al., 2001; Minobe, 2002; Hare and Mantua, 2000), much of the variance of physical variables lies in the interannual frequency band. The OCH addresses the impact of annual changes in the following manner. When a cold year occurs in a warm regime, the inertia of the large biomass of predatory adult fish maintains a top-down energy flow. If sufficiently large year-classes of piscivorous predators (e.g., pollock) result from one or two warm years in a cold regime, the control could switch from bottom-up to top-down. Variations in forage fish and pollock year-class strength can also occur within a regime as a result of the redistribution of their predators.