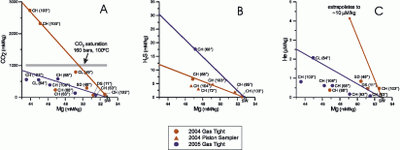
The analytical results for the 2004 and 2005 samples from NW Eifuku are summarized in Tables 1 and 2. By theory and observation, hightemperature hydrothermal fluids are nearly devoid of Mg, so pure "end-member" fluid compositions normally are estimated by extrapolating to zero Mg [Edmond et al., 1979; Seyfried, 1987; Von Damm, 1990]. However, even though in-line temperatures of ~103°C were measured during sampling, none of the samples collected at the Champagne site had a Mg concentration less than 43 mmol/kg, although several samples approach this value. As we will explain in the Discussion section, we do not believe that a high temperature zero-Mg endmember exists at the Champagne site. Rather than extrapolate to zero magnesium, which would yield unrealistically high temperatures and CO2 concentrations, we assign the value of 43 mmol/kg Mg to the undiluted 103°C vent fluid that exists at the seafloor. In adopting this approach, we assume that the range of Mg concentrations that we measure is due to entrainment of local seawater during sampling. In Figure 8, vent fluid properties are plotted versus Mg, and mixing lines are shown extrapolating the concentrations to the assumed end-member Mg value of 43 mmol/kg. By extrapolating to this value, we believe we are correcting each sample for seawater dilution during sampling.
Figure 8. Vent fluid concentrations for CO![]() , H
, H![]() S, and He versus Mg for NW Eifuku vent fluids. Vent designations are CH (Champagne), SD (Sulfur Dendrite), CL (Cliff House), and DS (Diffuse Site). The 2004 collections are shown in red; the 2005 collections are shown in blue. Fluid discharge temperatures are indicated in parentheses. All of these sites are near the Champagne vent field (see Figure 1b). Possible mixing lines are shown indicating end-member compositions for 2004 samples (red lines) and 2005 samples (blue lines). The solubility of CO
S, and He versus Mg for NW Eifuku vent fluids. Vent designations are CH (Champagne), SD (Sulfur Dendrite), CL (Cliff House), and DS (Diffuse Site). The 2004 collections are shown in red; the 2005 collections are shown in blue. Fluid discharge temperatures are indicated in parentheses. All of these sites are near the Champagne vent field (see Figure 1b). Possible mixing lines are shown indicating end-member compositions for 2004 samples (red lines) and 2005 samples (blue lines). The solubility of CO![]() in water at these conditions (160 bars, 100°C) is shown for comparison.
in water at these conditions (160 bars, 100°C) is shown for comparison.
Turning our attention at first to the 2004 results, based on an extrapolation to 43 mmol/kg Mg, the 103°C Champagne hydrothermal fluid contained a surprising ~3.0 moles/kg of CO![]() . This is an order of magnitude higher than any CO
. This is an order of magnitude higher than any CO![]() values previously reported for submarine hydrothermal fluids (Figure 9). As will be discussed later, we believe that this very high CO
values previously reported for submarine hydrothermal fluids (Figure 9). As will be discussed later, we believe that this very high CO![]() concentration in the vent fluid is the result of subsurface entrainment of liquid CO
concentration in the vent fluid is the result of subsurface entrainment of liquid CO![]() and/or CO
and/or CO![]() hydrate. The overall gas composition of the 2004 vent fluid was ~3000 mmol/kg CO
hydrate. The overall gas composition of the 2004 vent fluid was ~3000 mmol/kg CO![]() , ~12 mmol/kg H
, ~12 mmol/kg H![]() S, <0.2 mmol/kg CH
S, <0.2 mmol/kg CH![]() and H
and H![]() , and 0.01 mmol/kg
, and 0.01 mmol/kg ![]() He. Although we analyzed for CO, it was for the most part below our detection limit (Table 1). Concentrations of N
He. Although we analyzed for CO, it was for the most part below our detection limit (Table 1). Concentrations of N![]() , O
, O![]() , Ar, and Ne are also included in Table 1 as indicators of air or seawater contamination. Two of the samples collected in 2005 (H494-GT4 and H497-GT7) did suffer from air contamination on the basis of their N
, Ar, and Ne are also included in Table 1 as indicators of air or seawater contamination. Two of the samples collected in 2005 (H494-GT4 and H497-GT7) did suffer from air contamination on the basis of their N![]() and O
and O![]() concentrations, but not enough to compromise the other gas measurements. The Champagne vent fluids have lithium concentrations in the range of 20 to 26
concentrations, but not enough to compromise the other gas measurements. The Champagne vent fluids have lithium concentrations in the range of 20 to 26 ![]() mol/kg, significantly lower than the ambient seawater concentration (26.5
mol/kg, significantly lower than the ambient seawater concentration (26.5 ![]() mol/kg), and pH ranging from 3.4 to 4.8. The low end of measured pH of Champagne vent fluids is consistent with CO
mol/kg), and pH ranging from 3.4 to 4.8. The low end of measured pH of Champagne vent fluids is consistent with CO![]() buffering in the end-member fluid.
buffering in the end-member fluid.
Figure 9. Histogram comparing estimated end-member CO![]() concentrations for vent fluids from mid-ocean ridges [Kelley et al., 2004], the Okinawa Trough [Sakai et al., 1990a, 1990b], and NW Eifuku (this work). For the MOR and Okinawa Trough samples, the end-member concentrations were derived in the usual way by extrapolating to zero Mg. For the NW Eifuku samples a value of 43 mmol/kg was used for this end-member extrapolation (see text for explanation).
concentrations for vent fluids from mid-ocean ridges [Kelley et al., 2004], the Okinawa Trough [Sakai et al., 1990a, 1990b], and NW Eifuku (this work). For the MOR and Okinawa Trough samples, the end-member concentrations were derived in the usual way by extrapolating to zero Mg. For the NW Eifuku samples a value of 43 mmol/kg was used for this end-member extrapolation (see text for explanation).
Table 1. Gas Compositions for Vent Fluid and Liquid Droplet Samples From NW Eifuku
As discussed above, we were not able to collect an unfractionated sample of the liquid droplets in 2004. However, analysis of the one liquid droplet sample that we collected confirmed that the droplets were composed of >90% CO![]() , with the remaining gas assumed to be H
, with the remaining gas assumed to be H![]() S.
S.
As discussed above in the Methods section, determining H![]() S concentrations in these NW Eifuku samples was challenging because of the high gas content of the samples. Shipboard analysis of samples collected with the non-gas-tight PVC pistons shows a roughly linear trend of increasing H
S concentrations in these NW Eifuku samples was challenging because of the high gas content of the samples. Shipboard analysis of samples collected with the non-gas-tight PVC pistons shows a roughly linear trend of increasing H![]() S with decreasing Mg, with H
S with decreasing Mg, with H![]() S reaching approximately 4.5 mmol/kg in the water phase of the least diluted PVC piston samples (Figure 8b). However, because the PVC piston samples have lost significant gas volume in most cases, these are minimum values for H
S reaching approximately 4.5 mmol/kg in the water phase of the least diluted PVC piston samples (Figure 8b). However, because the PVC piston samples have lost significant gas volume in most cases, these are minimum values for H![]() S in the fluids. One gas-tight sample collected in 2004 (R793-GT5) was analyzed by sulfur chemi-luminescence gas chromatography at Atmospheric Analysis and Consulting, Inc., and this value lies on the mixing line through the highest of the PVC piston results (Figure 8c). This suggests that the end-member concentration (~12.5 mmol/kg H
S in the fluids. One gas-tight sample collected in 2004 (R793-GT5) was analyzed by sulfur chemi-luminescence gas chromatography at Atmospheric Analysis and Consulting, Inc., and this value lies on the mixing line through the highest of the PVC piston results (Figure 8c). This suggests that the end-member concentration (~12.5 mmol/kg H![]() S) derived from this mixing line (the red mixing line) represents the best estimate for the 2004 Champagne fluid composition. In all of the samples analyzed at AAC, H
S) derived from this mixing line (the red mixing line) represents the best estimate for the 2004 Champagne fluid composition. In all of the samples analyzed at AAC, H![]() S was the only sulfur species detected.
S was the only sulfur species detected.
During the return visit to NW Eifuku in 2005, while the Champagne site still had a constant flow of vent fluid and liquid droplets, there seemed to be slightly less activity than observed in 2004. During our first visits to the Champagne site in 2004, the vent fluid was discharging through several small white chimneys (see Figures 3a, 3b, and 3c). We were surprised to find that these chimneys, which were destroyed by the ROV during the 2004 sampling, had not re-grown during the intervening 18 months. As shown in Tables 1, 2, and 3 and in Figure 8, on the basis of "end-member" extrapolations, compared to 2004 the CO![]() concentration was lower in 2005, accompanied by higher H
concentration was lower in 2005, accompanied by higher H![]() S, lower He, and higher C/
S, lower He, and higher C/![]() He ratios. One of the major accomplishments of the 2005 Hyper-Dolphin dives was the successful collection of 4 uncontaminated samples of the liquid droplets (see Methods section above). The liquid droplets had a gas composition of ~98% CO
He ratios. One of the major accomplishments of the 2005 Hyper-Dolphin dives was the successful collection of 4 uncontaminated samples of the liquid droplets (see Methods section above). The liquid droplets had a gas composition of ~98% CO![]() , <0.01% CH
, <0.01% CH![]() and H
and H![]() , ~6 ppm He, and ~0.8% H
, ~6 ppm He, and ~0.8% H![]() S (see Tables 1 and 3). While this composition is similar to that of the vent fluids, the liquid droplets collected in 2005 have lower H
S (see Tables 1 and 3). While this composition is similar to that of the vent fluids, the liquid droplets collected in 2005 have lower H![]() S/CO
S/CO![]() and higher He/CO
and higher He/CO![]() ratios than the 2005 vent fluids (Table 3).
ratios than the 2005 vent fluids (Table 3).
Table 2. Isotope Ratios for Vent Fluid and Liquid Droplet Samples From NW Eifuku
Table 3. Estimated End-Member Compositions, Based on Extrapolating to a Mg Concentration of 43 mmol/kg
The helium in both the Champagne vent fluid and in the liquid droplets had an isotopic ratio of R/R![]() = 7.31 ± 0.05, a value typical of subduction zone systems (R =
= 7.31 ± 0.05, a value typical of subduction zone systems (R = ![]() He/
He/![]() He and R
He and R![]() = R
= R![]() ) [Poreda and Craig, 1989; Hilton et al., 2002]. During the 2003 water column surveys over NW Eifuku, helium samples were collected that allowed an estimate of the end-member helium isotope ratio based on the co-variation of [
) [Poreda and Craig, 1989; Hilton et al., 2002]. During the 2003 water column surveys over NW Eifuku, helium samples were collected that allowed an estimate of the end-member helium isotope ratio based on the co-variation of [![]() He] versus [
He] versus [![]() He]. This estimate gave R/R
He]. This estimate gave R/R![]() = 7.25 ± 0.4, in remarkably good agreement with direct measurements of the vent fluids (see Figure 10). Furthermore, the C/
= 7.25 ± 0.4, in remarkably good agreement with direct measurements of the vent fluids (see Figure 10). Furthermore, the C/![]() He ratio estimated from measurements of
He ratio estimated from measurements of ![]() CO
CO![]() and [
and [![]() He] in the water column plumes differed only by 10% from that determined from the vent fluids (Figure 10c). This suggests that reliable estimates of the ratios of certain vent fluid properties can be made from samples of the overlying water column plumes, even though these plumes typically contain only 0.1% or less of the pure vent fluid.
He] in the water column plumes differed only by 10% from that determined from the vent fluids (Figure 10c). This suggests that reliable estimates of the ratios of certain vent fluid properties can be made from samples of the overlying water column plumes, even though these plumes typically contain only 0.1% or less of the pure vent fluid.
Figure 10. Plots showing ![]() He,
He, ![]() He, and CO
He, and CO![]() data collected in the water column over NW Eifuku in 2003 (filled triangles) and 2004 (filled circles). (a) [
data collected in the water column over NW Eifuku in 2003 (filled triangles) and 2004 (filled circles). (a) [![]() He] and
He] and ![]() CO
CO![]() versus depth. The depth of the Champagne vent field is indicated for comparison. (b) [
versus depth. The depth of the Champagne vent field is indicated for comparison. (b) [![]() He] versus [
He] versus [![]() He] showing an estimate of the end-member
He] showing an estimate of the end-member ![]() He/
He/![]() He ratio based on a linear regression fit. Here R =
He ratio based on a linear regression fit. Here R = ![]() He/
He/![]() He and R
He and R![]() = R
= R![]() = 1.39 × 10
= 1.39 × 10![]() . (c)
. (c) ![]() CO
CO![]() versus [
versus [![]() He] showing a similar estimate of the end-member CO
He] showing a similar estimate of the end-member CO![]() /
/![]() He ratio.
He ratio.
Isotopic analysis of the CO![]() in the Champagne vent fluid gave
in the Champagne vent fluid gave ![]()
![]() C = -1.75‰, while the carbon in the liquid droplets was slightly heavier (
C = -1.75‰, while the carbon in the liquid droplets was slightly heavier (![]()
![]() C = -1.24‰). The C/
C = -1.24‰). The C/![]() He ratio ranged from 1.6 to 9.7 × 10
He ratio ranged from 1.6 to 9.7 × 10![]() for the Champagne vent fluids, and from 1.4 to 1.9 × 10
for the Champagne vent fluids, and from 1.4 to 1.9 × 10![]() for the liquid droplets (Table 2). A sample of the CO
for the liquid droplets (Table 2). A sample of the CO![]() from the Champagne site 103°C vent fluid was analyzed for radiocarbon at the CAMS facility at Lawrence Livermore National Laboratory. The result was
from the Champagne site 103°C vent fluid was analyzed for radiocarbon at the CAMS facility at Lawrence Livermore National Laboratory. The result was ![]()
![]() C = -998.7‰, corresponding to an age of 53450 ± 3200 years, or a fraction of modern carbon of only 0.0013 (see Table 2). Analyses of the 68°C vent fluid and of the liquid CO
C = -998.7‰, corresponding to an age of 53450 ± 3200 years, or a fraction of modern carbon of only 0.0013 (see Table 2). Analyses of the 68°C vent fluid and of the liquid CO![]() droplets yielded similar results. Thus the carbon in the Eifuku CO
droplets yielded similar results. Thus the carbon in the Eifuku CO![]() is "dead" (age ≥ 50,000 years).
is "dead" (age ≥ 50,000 years).
Return to previous section or go to next section